is liquid soap homogeneous or heterogeneous
Educator explains the observations. Mixtures contain two or more components.

Pin By Rachel Thornton On Chem 100 In 2021 Chemical Changes Physical Change Chemistry
Its an obviously heterogeneous mixture because there are two states or phases of matter.

. A homogeneous b heterogeneous c heterogeneous. Hydrogenation is a chemical reaction between molecular hydrogen H 2 and another compound or element usually in the presence of a catalyst such as nickel palladium or platinumThe process is commonly employed to reduce or saturate organic compoundsHydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule often an alkene. In science mixtures can fall under two broad categories.
Some are solid like brass and rocks or liquid like seawater and fruit juices or gas like air. It is a homogeneous mixture of two or more substances. Mixtures are of two types-homogeneous and heterogeneous.
In Module 1 you learned about solutions homogeneous mixtures. D colloidal system is a heterogeneous mixture. The dispersed particles are spread evenly throughout the dispersion medium which can be a solid liquid or gas.
Heterogeneous mixtureIn this form of mixture the composition of substance are not Oct 28 2019 Which of the following is a heterogeneous mixture. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. There are patches of dense liquid water falling down through the less dense air.
HOMOGENEOUS MIXTURES Milk is a mixture of liquid butterfat globules dispersed and suspended in water. Examples are starch solution and soap solution c Other name for homogeneous mixtures is solutions. Wash your hands with soap after completing the experiment.
C Soap bubbles formed by blowing air into soap solution. Using nonionic or zwitterionic detergents it is possible to select conditions that retain the proteindetergent micelles on the column. The statement not true for suspensions is a they are transparent b they.
The boiling point of A is 60C while that of B is 90C. What is a pure substance. What is a homogeneous mixture.
The component of the solution that is. The particles are spread evenly throughout the dispersion medium which can be a solid liquid or gas. Keep in mind that mixtures can involve the combination of.
Catalysis in chemistry the modification of the rate of a chemical reaction usually an acceleration by addition of a substance not consumed during the reactionThe rates of chemical reactionsthat is the velocities at which they occurdepend upon a number of factors including the chemical nature of the reacting species and the external conditions to which they are exposed. The particles in a colloid can be solid liquid or bubbles of gas. Of liquid dish soap is added to milk the surface tension of the milk is reduced.
Water striders to float on a water surface without becoming even partly submerged. It is because of the high vapour pressure of the liquid state of the solid. Surface tension is what allows objects with a higher density than water such as razor blades and insects eg.
In contrast when they cannot be mixed they are immisciblethey will form two separate layers called a heterogeneous solution. The liquid state is. Heterogeneous mixture a Homogeneous mixture.
The clear liquid which is left behind in the beaker after settling down of the sediments is called. Identify the suspension in the following a soap in water 6 milk in water c alcohol in water d saw dust in water. A homogeneous mixtureis a type of mixture that is considered to be the same throughout.
Solutions like apple juice are homogeneous mixtures. Liquid soap - Hand soap liquid dishwashing soap and liquid laundry detergent are solutions of various compounds in water. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible.
Because the dispersed particles of a colloid are not as large as those of a suspension they do not settle out upon standing. B Particles of a colloid can be seen by naked eye. Every sample of the mixture will show the same amounts of each substance.
That as the brown copper metal a pure substance and a solid reacts with the clear concentrated nitric acid a liquid and a mixture a reddish brown gas is given off and a blue liquid is formed the gas mixes in the atmosphere to form a mixture of gases with the air particles in this reaction matter is. To liquids powderliquid mixtures and slurries. As this occurs the fat butterfat.
Lemonade and gaseous solutions eg. Suggest a method to separate them. It appears that the fibers of the dispersing medium form a complex three.
A solution is made up of solute and solvent. Heterogeneous mixtures are not the same all throughout and they will settle out. NCERT Class 9 Science Lab Manual Solution Colloids Suspension Introduction Solution.
Heterogeneous mixture s include multi-phase substances different states of matter such as sand and water solid and liquid or carbonated drinks gas and liquid. Solutions can be solid solutions eg. The rainy atmosphere is a mixture of the air which is a gas with liquid rain droplets.
They have a uniform. To be able to mix the molecules of both liquids have to be. At liquidair interfaces surface tension results from the greater attraction of liquid.
Ion-exchange chromatography uses the difference in charge between proteindetergent micelles and homogeneous detergent vesicles. A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances. Two miscible liquids A and B are present in a solution.
C in a colloidal system dispersion medium is always in the liquid state. The medium that they are suspended in can be a solid liquid or gas although gas colloids cannot be. A colloid is a type of mixture intermediate between a homogeneous mixture also called a solution and a heterogeneous mixture with properties also intermediate between the two.
Homogeneous mixtures can be solid liquid gas or plasma mixtures. AWhat are the three general classes of matter. The separation can be done by the process of simple.
Homogeneous mixtures include single-phase substances the same state of matter such as coffee with creamer both liquid or sterling silver made with silver and copper. Homogeneous means that the two or more substances combine in such a way that the mixture is the same all throughout. The mixtures in which the constituting substances remain separated and one substance is spread throughout the other substance as small particles.
Colloid is a homogeneous mixture. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The variation in size may tell whether a mixture is homogeneous or heterogeneous.
Hielscher Ultrasonics specializes in the design and manufacturing of high power ultrasonic homogenizers for lab bench-top and production levelUltrasonic power is an effective and energy-efficient means to apply high shear and intense stress. These components may vary in size. Which of the following is a true solution.
This makes it a strong alternative to high shear mixers high pressure homogenizers and agitated bead. Gelatin sets on cooling because the hot aqueous mixture of gelatin coagulates as it cools and the whole mass including the liquid sets to an extremely viscous body known as a gel a colloid in which the dispersing medium is a solid and the dispersed phase is a liquid.

1 Composition Of Bar Of Soap And Liquid Soap Download Table

Phases And Classification Of Matter Chemistry I

Dancing Oobleck Educational Crafts Science For Kids Cool Science Experiments

Homogeneous Mixture Definition Examples Tutors Com

Examples Of Homogeneous Mixtures Solid Liquid And Gas
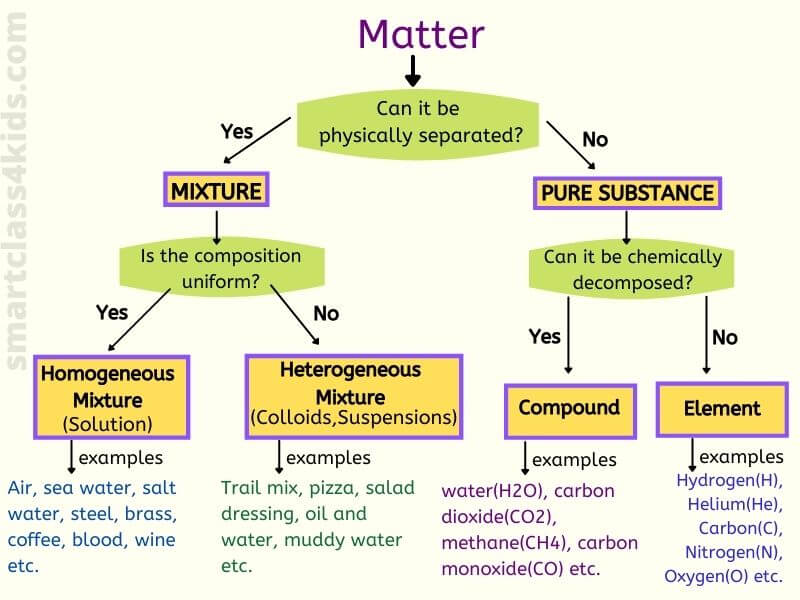
What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples

Physical Chemical Changes Printable Journal Notes Teacher Resources Teacher Resources Chemical Changes Student Journal

The Science Of Hair Part Four Using Ph Balance Properly For Hair Care Home Remedies For Asthma Natural Asthma Remedies Lemon Water

Homogeneous Mixture Definition Examples Tutors Com

5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends

Saturated Unsaturated And Superstaurated Solutions Classroom Charts Teaching Chemistry Teaching Science

Pin By Joseph Michel On Crystal Perfume Bottle S In 2021 Beautiful Perfume Bottle Crystal Perfume Bottles Beautiful Perfume

Benefits Of Jamaican Black Castor Oil For Skin Around The Eyes Url Http Castoroil Org Fb Fan Page Https Castor Oil For Skin Castor Oil Eye Makeup Remover

Delicious Can 39 T Wait For Easter So I Can Drink These Again Delicious Drinks Food

This Product Is My Calculating Speed Activity That Can Take Place In A Gym Field Hallway Or Classr Fun Math Activities Calculating Speed Science Lesson Plans

Elements Compounds And Mixtures Activity Foldable No Prep Scoot Game Quiz Matter Activities Elements Compounds Mixtures States Of Matter

Lego Mixture Pure Substances Opener Solutions And Mixtures Pure Products Lego


0 Response to "is liquid soap homogeneous or heterogeneous"
Post a Comment